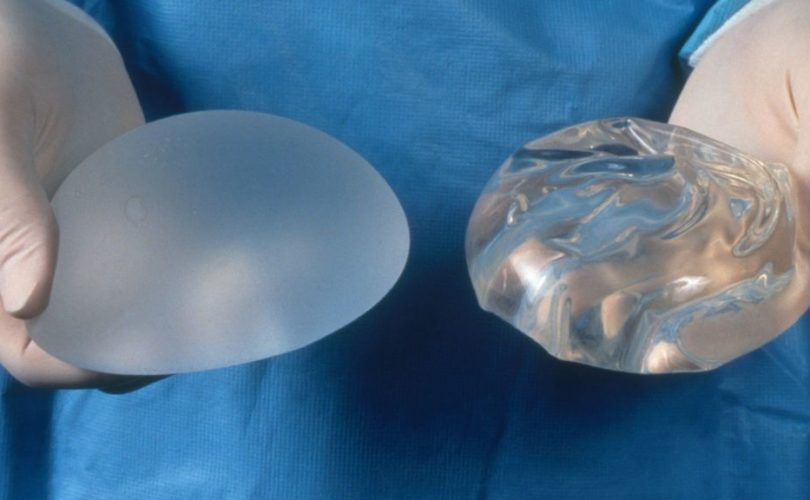
In recent years, some people have suspected that their breast implants have made them very ill with diseases such as:
rheumatoid arthritis
scleroderma
Sjögren’s syndrome
It is called “Breast Implant Illness”. There was not a lot of evidence for this phenomenon.
But new studies from different sources have found an association between silicone breast implants and certain autoimmune diseases.
These studies suggest that silicone breast implants potentially raise your risk of developing an autoimmune disease such as rheumatoid arthritis, Sjögren’s syndrome, scleroderma, and sarcoidosis.
The World Health Organization and the U.S. Food and Drug Administration have identified another possible cause for concern.
This relates breast implants to a rare cancer called breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
On May 2, 2019, the FDA finally issued a statement acknowledging breast implant illness and some of the systemic symptoms caused by silicone.
Earlier, on February 6, 2019, the FDA warned health care providers about the association of all kinds of breast implants and cancer.
In September 2018 one of the largest ever studies published shows breast implants ARE associated with rare diseases, autoimmune disorders, and other serious health problems.
Textured breast implants made by Allergan that have been linked to an unusual cancer are being recalled in the United States at the request of the Food and Drug Administration, and will also be recalled globally, the FDA announced.
The FDA decision, based on an increasing number of cases and deaths from the implant-associated cancer, lags far behind action in Europe, where the Allergan devices were effectively banned late last year.
Worldwide, 573 cases and 33 deaths from cancer have been reported, with 481 of the cases clearly attributed to Allergan Biocell implants, the FDA said. Of the 33 deaths, the agency said its data showed that the type of implant was known in 13 cases, and in 12 of those cases the maker was Allergan.
“The data regarding deaths was particularly informative of our decisions,” said Dr. Jeffrey Shuren, director of the FDA’s Center for Devices and Radiological Health.
The disease is anaplastic large-cell lymphoma, a rare cancer of the immune system. It is not breast cancer but develops in tissue around the implant.
In most cases, removing the implant and the scar tissue around it cures cancer, but if it is not detected early it can spread and kill the patient.
The condition has occurred with implants placed for cosmetic breast enlargement and with those used for reconstruction after mastectomy for breast cancer.
Despite the low incidence of the lymphoma, the FDA said in a safety communication for patients that “at the present time, we believe all individuals who are considering a breast implant of any type be informed of the risk of developing” the disease.
The Biocell textured implants carry a risk that is about six times that of other textured implants sold in the United States, the agency said.
Hundreds of thousands of women in the United States have Biocell implants, Dr. Binita Ashar, director of the FDA Office of Surgical and Infection Control Devices, said at a news briefing on Wednesday about the recall.
The main symptoms of the lymphoma are usually swelling and fluid accumulation around the implant.
A diagram from the F.D.A. showing how the cancer forms around the implant. The lymphoma is usually found near the breast implant, contained within the fibrous scar capsule or in the fluid surrounding the implant.
The disease is not in the breast tissue itself. Researchers have not been able to explain the link between the textured implants and the rare cancer.
The agency held two days of emotionally charged meetings about breast implants in March, hearing from women who had had lymphoma and others who had developed debilitating symptoms like pain and fatigue after receiving implants, which the FDA had acknowledged might occur. The lymphoma patients called for a ban on textured implants.
The recalled devices listed by the F.D.A. are Biocell products including:
Natrelle saline-filled breast implants, Natrelle silicone-filled breast implants, Natrelle Inspira silicone-filled breast implants, and Natrelle 410 highly cohesive anatomically shaped silicone-filled breast implants. The recall also includes tissue expanders used by patients before breast augmentation or reconstruction, including Natrelle 133 Plus tissue expander and Natrelle 133 tissue expander with suture tabs.
The FDA officials said they were considering changes to the labeling of breast implants, like adding a black-box warning to draw attention to the risks and requiring doctors and patients considering the surgery to go over a checklist to help women understand the benefits and risks of the devices.
The contents of the implant, silicone or saline, are not a factor in the lymphoma. The covering or shell of the implant, which can be either smooth or textured, is the key.
Textured implants, which have a slightly roughened surface that adheres to tissue and helps hold the device in place, have been singled out as the cause of the lymphoma.
Allergan’s textured implants account for only about 5 percent of the implants used in the United States but have been much more common in Europe, where they have already been recalled by many countries. Worldwide, 38 countries have banned textured implants.
When women decide to get breast implants for reconstruction after mastectomy or for breast augmentation, they should not be putting their lives at risk for lymphoma.
This recall would reduce the risk but not eliminate it, because some women with other types of implants also had the lymphoma.
It is well-know that any object put in our bodies can be rejected by the immune system. For example, we know that dogs that got implants have a higher risk of developing cancer.
The same is true for us. Any foreign objects inserted into our bodies can potentially trigger some reactions like cancer and autoimmune conditions.
I have witnessed dozen of women getting implants and starting to get sick soon after.
And when they are removed, their health improves to normal again.
Sometimes, the side effects can be subtle. It can take years to develop. I have seen women that started to have issues 20 or 30 years later and never suspect the implants.
At the end of the day, it is never a good idea to have implants in the body.
God bless y’all 😊
Dr. Serge